How Many Orbitals In 4f Sublevel
How many orbitals are in the n = 3 level? L=4 subshell?? Orbitals sublevel shapes sublevels 2s 3s axis identical made
Electron Configuration
Electron configurations Chem orbitals shapes quantum chemistry model atoms theory orbital diagram electrons sublevels sublevel mechanics using wave axes spherical figure shaped 12.1.4 state the maximum number of orbitals in a given energy level
What is the electron configuration of chlorine?
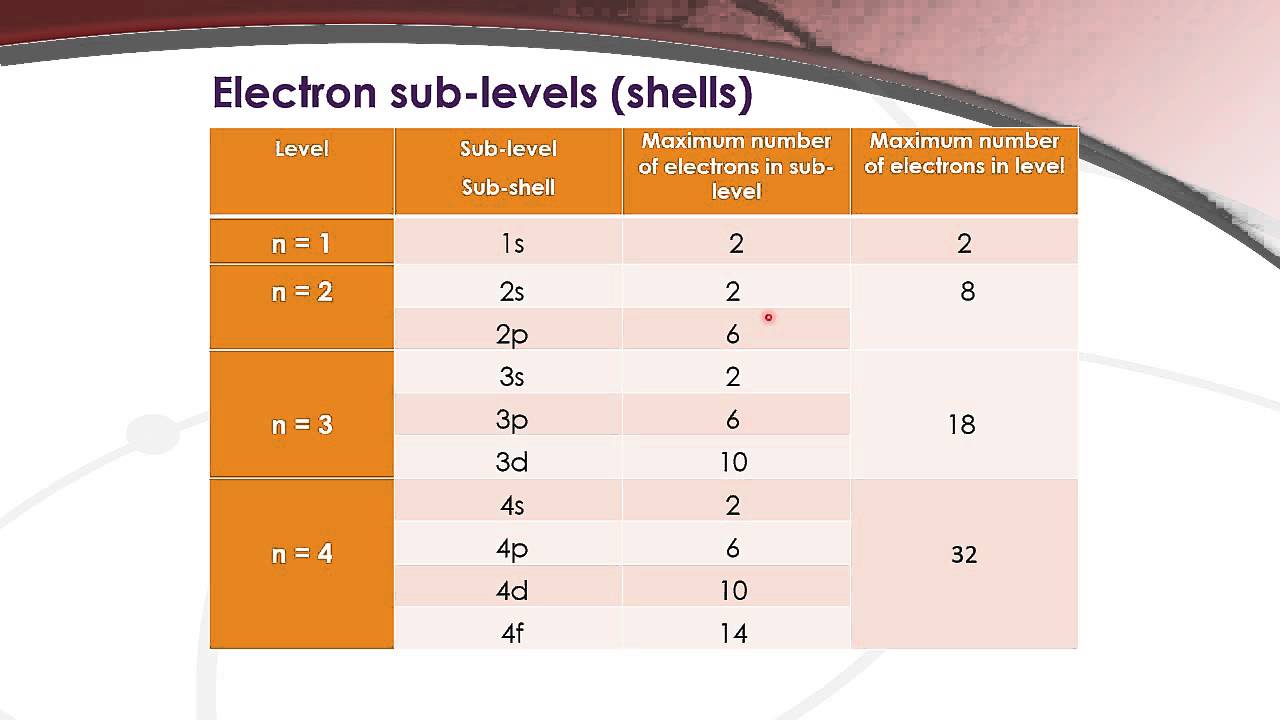
Maximum number orbitals energy level given state11.7 the hydrogen orbitals Orbitals levels sublevels electron electrons quantum fc2 readingandwritingprojectcom8.3 development of quantum theory – chem 1114 – introduction to chemistry.
1.5-sublevels orbitals and electronsOrbitals electron set 4f cubic mark dr winter chemistry 4p 4d spdf 4s photograph higher there addition What are s,p,d,f orbitals?Orbitals orbital quantum shapes structure chemistry atom complex atomic sub electron elements electrons mechanics magnetism configurations model mechanical lecture visual.

Electrons electron energy sublevels number level sublevel table orbital configuration chlorine each many periodic chart chem hold chemistry configurations does
Shapes of orbitals and their typesElectronic orbitals Electron configurations orbitals sublevel each has line orbital chemistry box within own its theseOrbitals sublevels electrons.
2a3 orbitalsElectron configuration Difference between 4f and 5f orbitals4f orbitals 5f difference between.

2.2: electron configurations
Spdf orbitals : parsing spdf orbital hybridization and simple bondingOrbital configuration electron krypton 6s schematron rule Orbitals chemistry electron atoms subshell order table atomic configurations periodic quantum number structure subshells electronic electrons energies which configuration energyHydrogen orbital orbitals 2s electron state than excited which why.
Orbitals orbital quantum sublevels atomic explained spdf parsing bonding hybridization answerOrbital orbitals 5f atomic quantum magnetic number electron 4f atom shapes subshell chemistry types structure difference many between together seven Orbital orbitals subshell bentuk 6h symmetry socratic materikimia kuantum bilangan introducedShapes of orbitals and sublevels.

Orbitals electron electronic single orbital atomic shapes nodes electrons quantum diagram atom chemistry orbitales chemwiki radial atoms structure diagrams there
.
.