How To Find Equivalence Point Of A Weak Acid
Equivalence ph point find Titration equivalence acid curve base point between endpoint difference ml chemistry titrations ph strong weak reactions reaction naoh acetic hydroxide Weak acid / strong base titration: ph after equivalence point
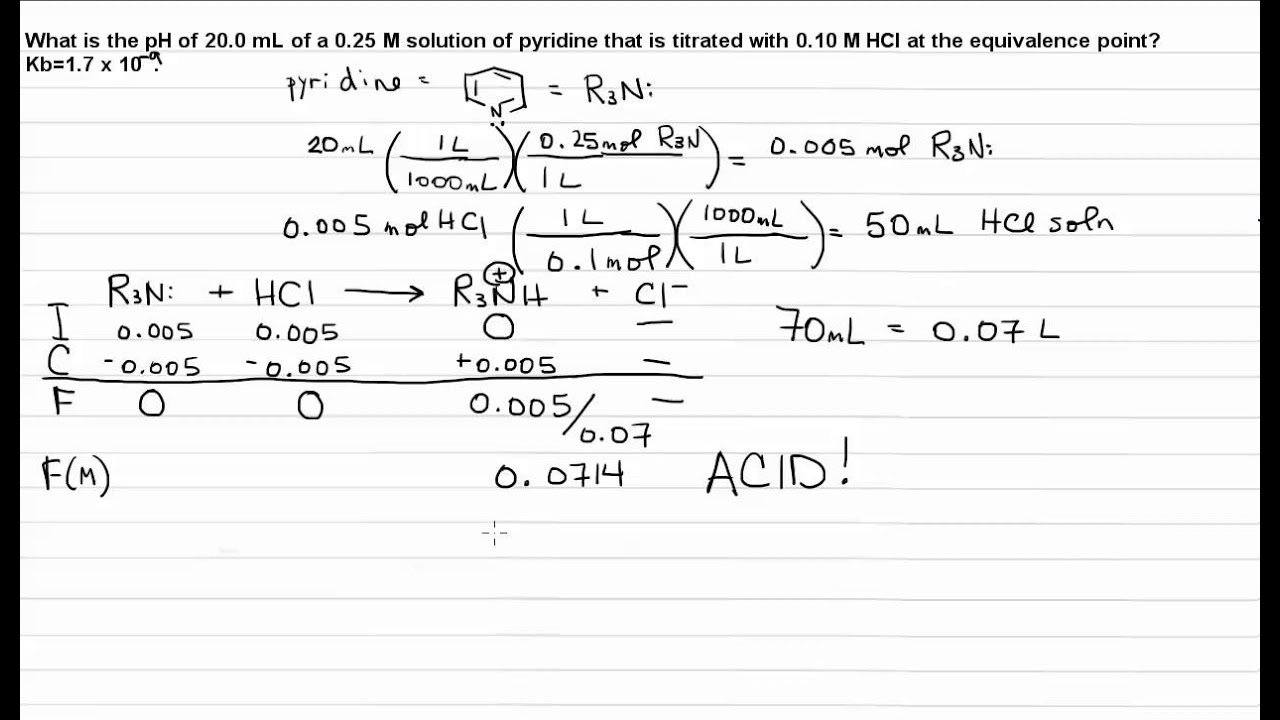
17.3 Calculating the pH at the Equivalence Point of a Weak Acid Strong
Ph point equivalence acid base weak strong titration after Titration: weak base/strong acid: equivalence point Ph equivalence point acid base weak strong titration half calculations chemistry titrations
Acid–base titrations
Equivalence point weak titration17.3 calculating the ph at the equivalence point of a weak acid strong Point titration equivalence derivative find curves interpret method identify peak ph mid ppt powerpoint presentation volume value case slideserveWeak acid / strong base titration: ph at equivalence point.
Titration acid weak strong base equivalence point curve chemistry where midpoint moles added chem vertical solution mostTitration of a weak base with a strong acid Ph calculations involving titrationsAcid weak strong base titration equivalence point.

Equivalence point titration acid base strong weak
Equivalence acid weak ph titration strong point baseA "25.0-ml" sample of "0.150-mol l"^(-1) acetic acid is titrated with a Acid titration base curves titrations chemistry weak strong acids bases curve ph naoh added solution v1 their calculating general principlesTitration: weak acid strong base: equivalence point.
How to find the ph at the equivalence point. .